The distribution of electrons in a multielectronic atom is based on the principle of the minimum of energy, the principle of V. Papuli, the rule of F. Hund and the rule of Klekkovsky.
The principle of the minimum of energy:
The electron is primarily located within the electronic sublayer with the lowest energy.
First, orbitals are filled, characterized by the smallest potential energy.
Powli principle:
There can be no two electrons in the atom, in which all four quantum numbers would be the same.
Consequently, each orbital characterized by certain values \u200b\u200bof N, ℓ and M ℓ can be occupied by no more than two electrons whose backs have opposite signs. Such electrons are called parsen.
Using the principle of Pauli, you can calculate which maximum number of electrons can be at each level and superts, i.e. Determine the level capacity and sublevel.
The number of orbitals on sublevels is determined by the number of possible values \u200b\u200bof the magnetic quantum number, i.e. 2ℓ + 1 Value. Since each orbital (a certain value of quantum numbers n, ℓ and m ℓ) can accommodate two electrons with different spin values, then the maximum number of electrons that can be placed on this suite is: 2 (2ℓ + 1). The maximum number of electrons that can be at a given level is characterized by the main quantum number n, equal to 2N 2.
Hund rule:
In the most stable state of the atom, the electrons are placed within the electronic sublayer so that their total spin is maximum. M. is a symbol of the Nableronons, which can be at the given level, characterized by the main quantum number
For example, a nitrogen atom at the external electron level is two and three P-electrons. Here, electrons in orbitals are depicted by arrows directed up or down depending on the sign of the spin quantum number. The S-S-believer contains the only orbital on which there are two electrons with opposite spins in accordance with the principle of Pauli. In accordance with the rule of Hinda, the minimum of energy will have configuration A, in which on each of the three p-orbitals will be one electron with the same directed spins, so the total spin will be maximized (± 3/2), and not in which the total Spin less (± 1/2):
The first rule Clakovsky:
The electron has the lowest energy on the electronic sublevel, where the sum of the main and orbital quantum number is minimal.
E \u003d min with n + ℓ \u003d min
In accordance with the first rule of Clekkovsky, the filling of sublayer electrons occurs in the order of sublayers with the minimum value of the amount (n + ℓ) to the sublayers in large values \u200b\u200bof N + ℓ.
If the sum (n + ℓ) is the same for the electronic submarkets under consideration, with the distribution of electrons is used the second rule Clakovsky:
Electron has the lowest energy on sublevels with the smallest value of the main quantum number.
4.5 Electronic Atomic Structure and Periodic Element System
At ℓ \u003d 0, i.e. On S-supro, there is only one orbital, which is usually depicted in the form of a cell. In the n atom, the only electron is at the lowest possible energy states, i.e. On the s-supro bellows of the first electronic layer (on 1s-pion). The electronic structure of the N atom can be submitted by the scheme:
In the helium atom, the sequence number of which in the periodic system (or the nucleus Z) is 2, the second electron is also in 1s state. Electronic structure of the helium atom:
This atom completes the filling of the K-layer nearest to the kernel and thus the construction of the first period of the element system is completed.
The methods of describing the electronic shells considered for atoms H and HE are called electron-graphic formulas (orbital images are depicted in the form of cells) and electronic formulas (the lines are denoted by letters, and the number of electrons to them is indicated by the upper index).
In the following helium of lithium element (z \u003d 3), the third electron can no longer be located on the orbital k-layer. The K-layer is called the first electronic layer of the atom.: This would contradict the principle of Pauli. Therefore, it occupies the S-state of the second energy level (L-layer. L-layer is called the second electronic layer of atom., N \u003d 2). Its electronic structure is recorded by 1S 2 2S 1 formula. The electronic structure of the following beryllium and boron follows it corresponds to the formula 1S 2 2S 2 and 1S 2 2S 2 P 1, which corresponds to the electron graphic formulas in the diagram:
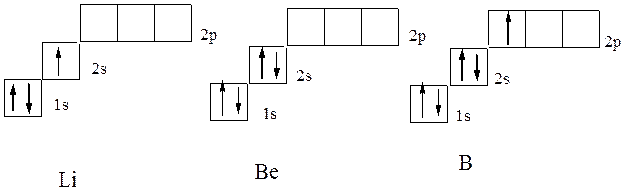
The abbreviated form of the electronic structure of the atom is used. At the same time, the structure of external blank levels is described, and instead of the electronic structure of the inner layers, the symbol of the inert gas is indicated in brackets, the electronic structure of which corresponds to the remaining part of the electronic formula. So, for the elements of the second period, the first electronic level (n \u003d 1) corresponding to the helium atom is fully filled. The electronic formula in the abbreviated version of atoms: lithium - 2s 1, beryllium version of the atom of the lithium atom: so for the elements of the second period, the electronic structure of Koto2S 2, boron - 2S 2 2p 1.
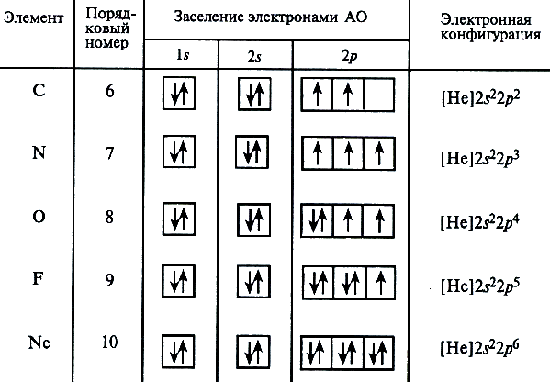
For a carbon atom, three possible schemes of filling electron shells can already be assumed in accordance with electronics and graphic formulas:
An analysis of the atomic spectrum shows that the latter scheme is correct. This procedure for placing electrons in the carbon atom represents a special case of a general pattern expressed by the Hinda rule: the stable state of the atom corresponds to such a distribution of electrons within the energy sublayer, in which the absolute value of the total spin of the atom is maximum. The electronic structure for the nitrogen atom (Z \u003d 7) is presented above.
Then the pairs of electrons on 2P orbitals begins. Electronic formulas of the remaining atoms of the second period:
O 1S 2 2S 2 2P 4; F 1S 2 2S 2 2P 5; NE 1S 2 2S 2 2p 6
The neo atom end filling out the second energy level, and the construction of the second period of the system of elements is completed.
The third period, similar to the second, begins with two elements (Na, Mg), in which electrons are placed on the S-pionery of the outer electronic layer. Such elements are called s-elements. Speaking differently, they belong to the S-elements family. (i.e., they relate to the S-family of elements). Then the six elements are followed (from Al to ar), which have a p-sublayer of an external electron layer. These are the atoms of p-elements (belong to the P-family). The structure of the outer electronic layer of the corresponding elements of the second and third periods is similar. In other words, with an increase in the charge of the kernel, the electronic structure of the outer layers of atoms is periodically repeated. However, the electronic structure of atoms determines the properties of the elements and their compounds. This is the essence of the Periodic Law: the properties of the elements and them formed by them simple and complex substances are in periodic dependence on the charge of the kernel.
The argon atom remains unoccupied by all orbitals 3D-sublevels. However, the following argon elements - potassium and calcium - the filling of the 3rd electronic layer is temporarily stopped, and the 4th layer S-su-layer begins to form. Such an order of filling follows from the first Clakovsky rule. Consequently, the 4S-sublayer (N + ℓ \u003d 4) must be filled earlier than 3D (n + ℓ \u003d 5). For the scandium atom, the question arises: which of the sublevels should be filled - 3D or 4P, because The sum N + ℓ is the same for them and equal to 5. In such cases, the completion order is determined by the second rule. The first and second Clakovsky rules are often not divided, but are considered to be one joint rule of the Clachkovsky rule, according to which the same amounts of the amount (N + ℓ) are filled in in order of increasing the main quantum number N. Filling a 3D sublayer occurs in ten elements from SC to Zn. These are D-elements atoms. Then the formation of 4P-suite begins (P-elements from Ga to Kr). As well as the atoms of the preceding noble gases - neon and argon - the crypton atom is characterized by the structure of the outer electronic layer NS 2 NP 6.
The fifth period is formed similarly.
In the sixth period, after filling, the 6S sublayer begins the filling 4f-suite, and the atoms of the F-elements are followed. Due to the fact that their external is the sixth level, and the electrons consistently occupy the 4th level, which is much closer to the kernel, the chemical properties of all these F-elements are close to Lanthan, so they are often called lanthanoids (in 7th The period F-elements are called actinoids). After 4F, 5D is filled in and finally, the 6P sublayer, which completes the construction of the sixth period. The seventh period is not completed, because Elements with a large charge of the kernel are very unstable (nuclear reactions are easily flowing).
The order of filling the sublevel in accordance with the Rules of Clakovsky can be written as a sequence: 1s → 2s → 2p → 3S → 3P → 4S → 3D → 4P → 5S → 4D → 5P → 6S → 4F → 5D → 6P → 7S → 5F → 6D → 7P. However, for some elements, this sequence is broken, i.e. From the rules of Clakovsky there are exceptions. At the CR, CU, NB, Mo, RU, RH, PD, AG, PT, AU atoms, there is a "failure" of an electron with an external layer S-su-supro-layer, which leads to an energetically more stable state of an atom in Most of the corresponding fully (CU, PD, AG, PT, AU) or half (CR, MO) filled with D-subframe. For example, the electronic formula of the copper atom has the form: Cu 1S 2 2S 2 2P 6 3S 2 3P 6 3D 10 4S 1, i.e. One of the two 4S electrons "fails" on a 3D-sublevel. It should be noted by palladium, whose "fall" two electrons: PD 1S 2 2S 2 2P 6 3S 2 3P 6 4S 2 4P 6 4D 10 5S 0. The second type of exceptions from the Clekkovsky rule is that one electron is located on the 5D sublayer before filling the 4f-suite: LA 1S 2 2S 2 2p 6 3S 2 3P 6 34 4S 2 4P 6 4D 10 4F 0 5S 2 5P 6 5D 1 6S 2. The next element (cerium) 5D-sudes is released, and both electrons are located on 4f-suite: CE 1S 2 2S 2 2P 6 3S 2 3P 6 3D 10 4S 2 4P 6 4D 10 4F 2 5S 2 5P 6 5D 0 6S 2. Similarly, in the 7th period of actinium, the latter of electrons is located on a 6D pioneer (and not 5f, as it should be according to the rules of Clekkovsky).